Background: Diffuse large B cell lymphoma (DLBCL) is the most common subtype of aggressive non-Hodgkin lymphoma (NHL) and is curable with first-line conventional chemotherapy, but a significant fraction of patients relapse or do not respond to initial therapy. There are a variety of treatments available for relapsed/refractory (RR) disease, including platinum-based salvage chemotherapy often used historically both with or without autologous stem cell transplant, and chimeric antigen receptor T-cell (CAR-T) therapy emerging more recently as a second-line option. We report survival data following 2 nd and 3 rd line therapy for RR DLBCL in a large contemporary prospective cohort.
Methods: We selected patients (pts) from the Lymphoma Innovations ORIEN Network (LION) with diffuse large B-cell lymphoma, not otherwise specified and high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements (“double-hit” lymphoma) who had previously received R-CHOP chemotherapy. All patients had signed written consent. Clinical data were abstracted from medical records using standardized protocol. Overall survival was estimated using Kaplan-Meier and defined as time from start of 2 nd (OS2) or 3 rd (OS3) line treatment until death, censoring patients alive at last follow-up. Comparisons of OS were performed using the log-rank test.
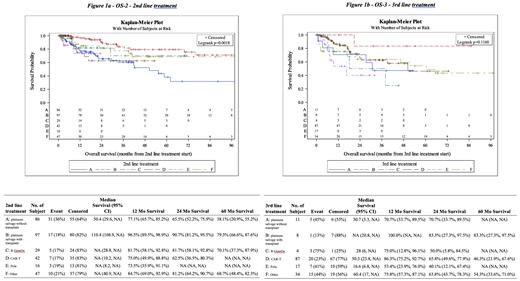
Results: A total of 328 patients with RR DLBCL who received 2 nd line treatment were included, 177 of whom went on to receive 3 rd line therapy. Baseline characteristics included male sex in 205 pts (65%), stage III/IV disease in 244 pts (81%), and extranodal involvement in 183 pts (63%). Second line treatments included platinum-based salvage chemotherapy both with (99 pts) and without (88 pts) autologous stem cell transplant, rituximab plus gemcitabine and oxaliplatin (30 pts, grouped distinctly from platinum-based salvage chemotherapy), CAR-T therapy (43 pts), polatuzumab vedotin (17 pts), and other classes of treatment (51 pts). Median OS2 for 2 nd line platinum-based salvage chemotherapy with autologous transplant was 110.4 months compared with 50.4 months for platinum-based salvage chemotherapy without transplant. OS2 for platinum-based salvage chemotherapy with autologous transplant was 96.5% (95% CI 90-99) at 12 months, 90.7% (95% CI 81-96) at 24 months, and 79.3% (95% CI 67-88) at 60 months compared with 77.1% (95% CI 66-85) at 12 months, 65.5% (95% CI 52-76) at 24 months, and 38.1% (95% CI 21-55) for platinum-based salvage without transplant. OS2 for rituximab plus gemcitabine and oxaliplatin was 81.7% (95% CI 58-93) at 12 months, still 81.7% (95% CI 58-93) at 24 months, and 70.1% (95% CI 37-88) at 60 months. OS2 for CAR-T cell therapy was 75% (95% CI 50-89) at 12 months and 62.5% (95% CI 36-80) at 24 months, it could not be calculated at 60 months. OS2 for polatuzumab vedotin was 73.5% (95% CI 36-91) at 12 months and could not be calculated at 24 or 60 months. OS3 for platinum-based salvage chemotherapy with autologous transplant was 100% at 12 months and 83.3% (95% CI 27-98) at 24 months compared with 70.7% (95% CI 34-90) at both 12 and 24 months for platinum-based salvage without transplant. OS3 for rituximab plus gemcitabine and oxaliplatin was 75% (95% CI 13-96) at 12 months and 50% (95% CI 6-85). OS3 for CAR-T therapy was 86.3% (95% CI 75-93) at 12 months, 65.8% (95% CI 50-78) and 46.3% (95% CI 22-68) at 60 months. OS3 for polatuzumab vedotin was 53.4% (95% CI 24-76) at 12 months and 40.1% (95% CI 12-67) at 24 months.
Conclusions: We observed a statistically significant difference by log-rank in OS2 in patients receiving platinum-based salvage chemotherapy followed by autologous stem cell transplant compared to other therapies in a contemporary cohort of pts with RR DLBCL. This difference is likely driven in part by transplant being a surrogate marker for efficacy of salvage chemotherapy. OS3 was also highest at 12, 24, and 60 months in patients receiving platinum-based salvage chemotherapy followed by transplant. Other therapies including CAR-T cells, polatuzumab vedotin, and rituximab plus gemcitabine and oxaliplatin did not demonstrate significantly different rates of overall survival in the 2 nd or 3 rd line setting.
Disclosures
Maddocks:BMS: Consultancy, Research Funding; AbbVie: Consultancy; Incyte: Consultancy, Honoraria; ADC Therapeutics: Consultancy; AstraZeneca: Consultancy, Research Funding; Gilead/Kite: Consultancy; Genentech: Consultancy; GenMab: Consultancy; Janssen: Consultancy, Honoraria; Morphosys: Consultancy; Pharmacyclics: Consultancy, Research Funding; Epizyme: Consultancy; BeiGene: Consultancy; Eli Lilly and Company: Consultancy; Seattle Genetics: Consultancy; Novartis: Research Funding; Merck: Research Funding; Celgene: Consultancy, Research Funding. Romancik:Astra Zeneca: Consultancy; KITE: Consultancy. Stephens:AbbVie: Consultancy; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy; Bristol-Myers Squibb: Consultancy; Celgene: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Lilly: Consultancy; Novartis: Research Funding. Pinilla-Ibarz:AbbVie: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; Secura Bio: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Lilly: Consultancy, Speakers Bureau; Genentech: Speakers Bureau. Chavez:Karyopharm: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Genmab: Honoraria; Epizyme: Speakers Bureau; Cellectar: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra Zeneca: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Research Funding; Lilly: Honoraria; Merck: Research Funding; Morphosys: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees. Matasar:Regeneron: Honoraria, Other: Stipends; Pharmacyclics: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Celegene: Honoraria, Other: Stipends; Epizyme: Other: Stipends; Kite: Honoraria, Other: Stipends; Immunovaccine Technologies: Honoraria; Seagen: Honoraria, Other: stipends; ADC Therapeutics: Consultancy, Honoraria, Other: Stipend; Merck: Current equity holder in private company; AstraZeneca: Honoraria, Other: Stipend; Bayer: Consultancy, Honoraria, Research Funding; BMS: Honoraria, Other: Stipend; Juno: Consultancy; Genentech, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria; Teva: Consultancy; Immunovaccine Technologies: Research Funding. Portell:Jansen: Honoraria; Merck: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Acerta/AstraZeneca: Research Funding; SeaGen: Research Funding; Loxo/Lilly: Research Funding. Cohen:Novartis: Research Funding; BMS/Celgene: Research Funding; Genentech: Research Funding; BioInvent: Research Funding; Lam Therapeutics: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Farooq:MorphoSys: Consultancy; Kite, a Gilead Company: Honoraria; Caribou: Consultancy, Honoraria; Regeneron: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal